Fluorine chemistry at extreme conditions: Possible synthesis of $HgF_4$
DOI:
https://doi.org/10.4279/pip.110001Keywords:
fluorine chemistry at extreme conditions, mercury transition metal behavior, diamond anvil cell, high pressure, infrared spectroscopy, x-ray photochemistryAbstract
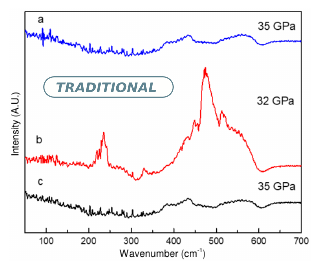
By irradiating a pressurized mixture of a fluorine-bearing compound ($XeF_2$) and $HgF_2$ with synchrotron hard x-rays (>7 keV) inside a diamond anvil cell, we have observed dramatic changes in the far-infrared spectrum within the 30-35 GPa pressure range which suggest that we may have formed $HgF_4$ in the following way: $XeF_2 \xrightarrow{hv} Xe + F_2$ (photochemically) and $HgF_2 + F_2 \rightarrow HgF_4$ (30 GPa < P < 35 GPa). This lends credence to recent theoretical calculations by Botana et al. that suggest that Hg may behave as a transition metal at high pressure in an environment with an excess of molecular fluorine. The spectral changes were observed to be reversible during pressure cycling above and below the above mentioned pressure range until a certain point when we suspect that molecular fluorine diffused out of the sample at lower pressure. Upon pressure release, $HgF_2$ and trace $XeF_2$ were observed to be remaining in the sample chamber suggesting that much of the $Xe$ and $F_2$ diffused and leaked out from the sample chamber.
Received: 29 October 2018, Accepted: 18 January 2019; Edited by: A. Goñi, A. Cantarero, J. S. Reparaz; DOI: http://dx.doi.org/10.4279/PIP.110001
Cite as: M Pravica, S Schyck, B Harris, P Cifligu, E Kim, B Billinghurst, Papers in Physics 11, 110001 (2019).
This paper, by M Pravica, S Schyck, B Harris, P Cifligu, E Kim, B Billinghurst, is licensed under the Creative Commons Attribution License 4.0.
Downloads
Published
How to Cite
Issue
Section
License
Authors agree to the PIP Copyleft Notice













